Crystal Lattices and Unit Cells :
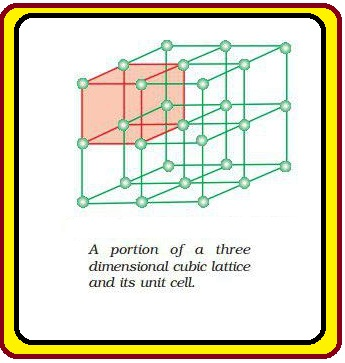
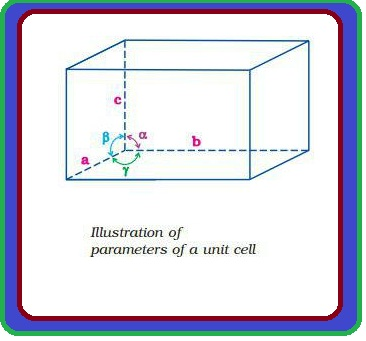
`color{green}("Crystal Lattice :")` The diagrammatical representation of three dimensional arrangement of constituent particles in a crystal is called crystal lattice. In this each particles is depicted as a point. .
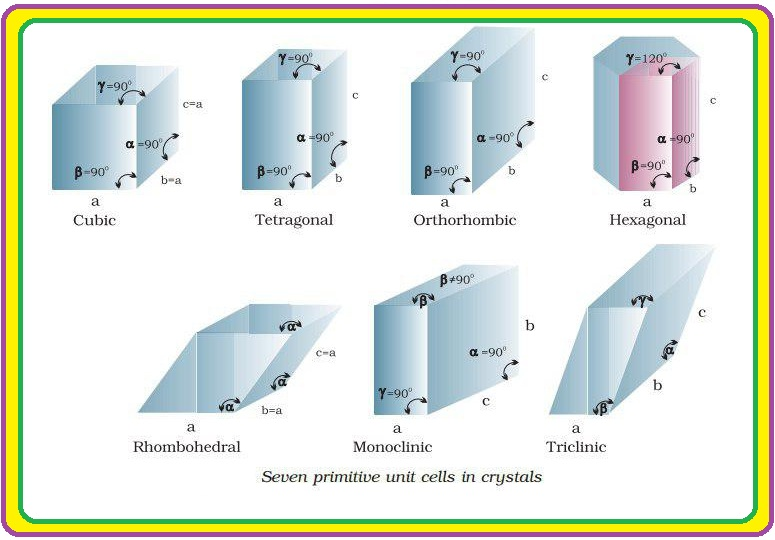
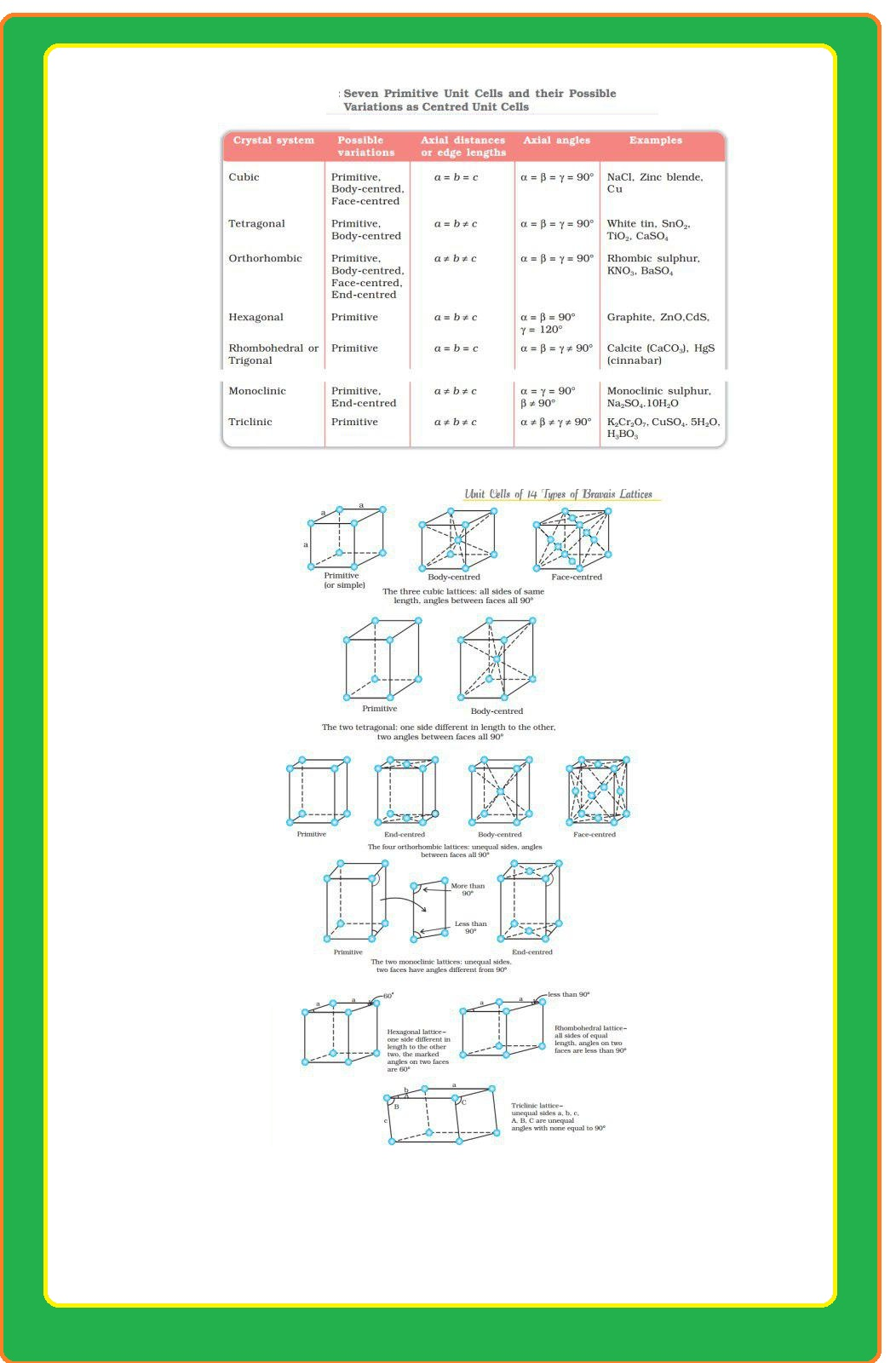
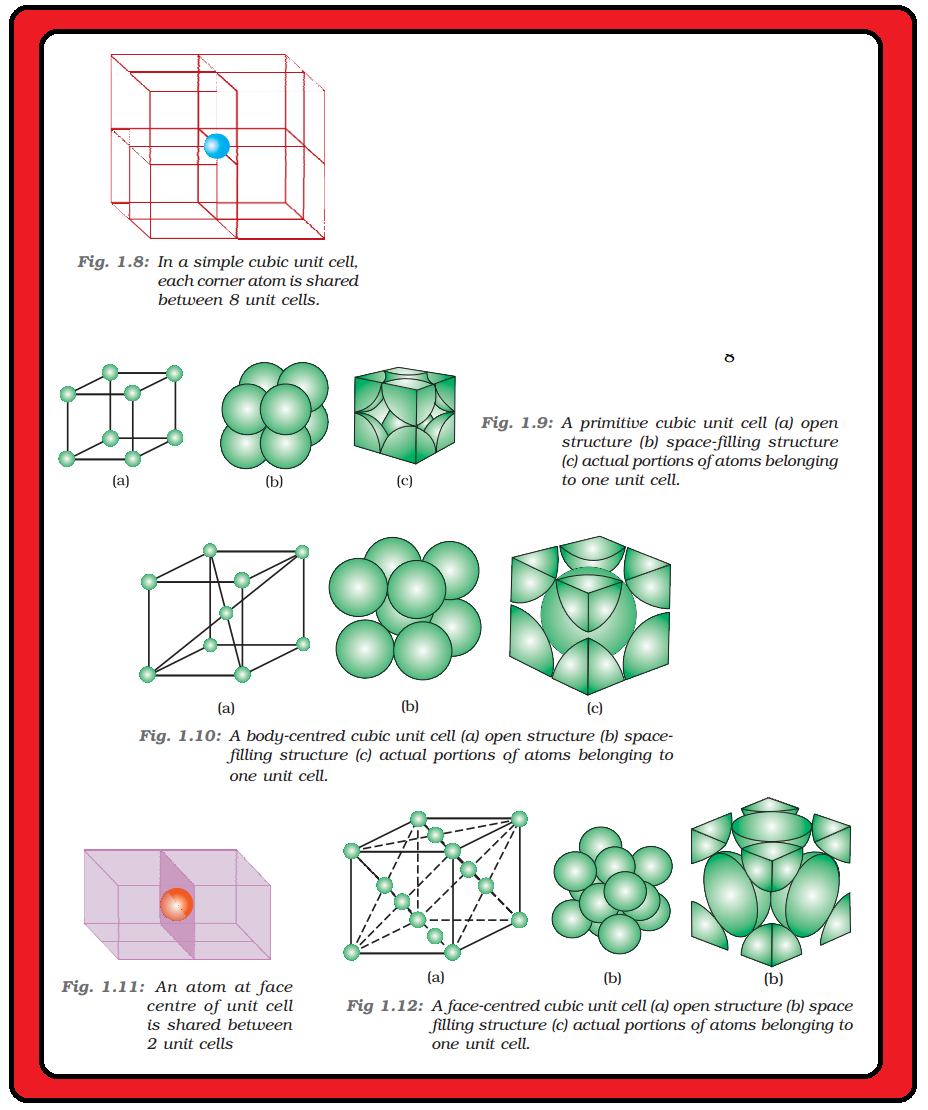
`color{green}("Bravias Lattices :")` There are only 14 possible three dimensional lattices. These are called Bravias Lattice.
`color{green}("Bravias Lattices :")` There are only 14 possible three dimensional lattices. These are called Bravias Lattice.